is metal alloys homogeneous or heterogeneous
Download and print the black and white pdf. Corrosion of boiler metal Caustic Embrittlement and Priming and Foaming.

Pin On Metallurgy Welding Engineer
The heterogeneous mixture homogeneous mixture worksheet with answer key is below.

. The opposite of heterogeneous mixtures is homogeneous mixtures. What Are Examples of Chemical Properties. Single Crystals Pure Element Single.
Due to the distinct design concept these alloys often exhibit unusual properties. Metal alloys are examples of solidsolid solutions. Explanation and Examples of Physical Properties.
The components of homogeneous mixtures are not physically distinct. The metal atom utilization in homogeneous molecular catalysts can reach 100 a figure that may be orders of magnitude higher than that of heterogeneous catalysts. Mixtures having a uniform composition all through the substance are called Homogeneous Mixtures.
The prefix homo indicates sameness. On the basis of the explanation from Liu 27 SSHC is defined as the catalyst with one or more atoms as the single site in which each site exists on its own without any interference. Mild or total oxidations dehydrogenation hydrogen transfer 18 O 2 16 O 2 and deuterium-alkane isotopic exchange metal deposition water detoxification gaseous pollutant removal etc.
Once mixed you cant easily separate the lemon juice from the water. Single atom catalysts hold the potential to significantly impact the chemical and energy industrial sectors. Examples of 10 Balanced Chemical Equations.
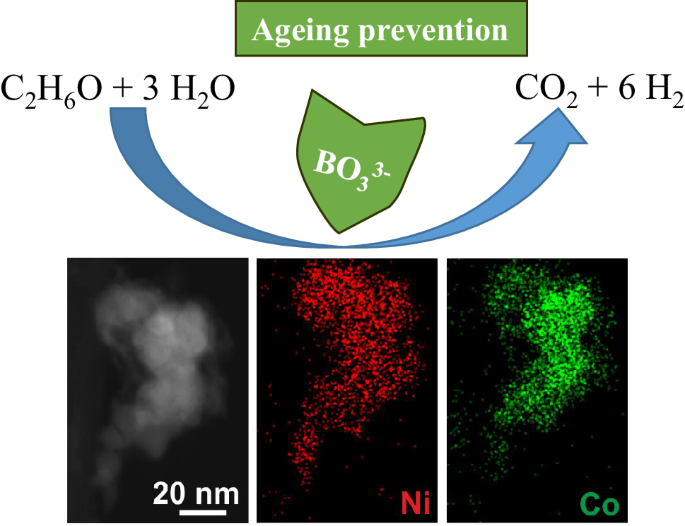
We found that the MCINPs were perfectly coherent with the matrix from transmission electron microscopy TEM. A heterogeneous mixture consists of visibly different. Homogeneous and heterogeneous are two different words that we can distinguish by the context in.
A homogeneous mixture has the same uniform appearance and composition throughout its mass. For example steel is. Which indicated a homogeneous and fully recrystallized microstructure with a random distribution of various oriented grains in both AI7Ti7 and Al8Ti6 alloys figs.
Is It Possible To Turn Lead into Gold. What Caustic Soda Is and Where You Can Get It. One example of an alloy is steel which is made from a mixture of iron and carbon.
In molten state alloys are homogeneous and in solid state they may be homogeneous or heterogeneous. Heres Why Salt Melts Ice. These are mixtures that are uniform throughout their composition.
Improving the strength of a metal alloy is hard to do without sacrificing the ductility. Is pizza a homogeneous or heterogeneous mixture. Solid in solid.
High-entropy alloys HEAs are alloys with five or more principal elements. An example of a homogeneous mixture would be something like lemonade. Distinguishing between homogeneous and heterogeneous mixtures is a matter of the scale of sampling.
Alloys Alphabetical listing of Alloys. Proteomics HPLC Solvents and additives. Heterogeneous photocatalysis is a discipline which includes a large variety of reactions.
The distinction between heterogeneous and homogeneous mixtures is a matter of magnification or scale. Although the Al-Si alloys are beneficial their tensile strength is modest and their ductility is low 4. Sterling silver - This common metal is actually an alloy of silver and.
The applications of both homogeneous and heterogeneous processes in carbon capture and storage were investigated in recent years and the focus now is on the conversion of CO2 into useful chemicals and compounds. In this case the. Alloys can be further classified as homogeneous consisting of a single phase heterogeneous consisting of two or more phases or intermetallic where there is no distinct boundary between phases.
Alloys homogeneous An alloy is a mixture of elements that has the characteristic of a metal. Many homes have bronze door knockers light fixtures or other design elements. The worksheet gives common examples of mixtures in addition to some pure un-mixed substances.
32 Catalysis Catalyst Positive catalyst Negative catalyst Definition Types of catalysis Homogeneous and Heterogeneous Promoter Catalyst poison Definition Characteristics of a catalyst Industrial applications. It was established that CO2 can undergo cycloaddition reaction with epoxides under the influence of special catalysts to give cyclic carbonates which can be used as value. Nitinol Nickel Titanium Shape Memory Alloys.
Think of a pizza that has a topping of cheese tomato mushrooms and peppers the topping is a mixture. Heterogeneous mixtures are generally seen in solid liquid and gases. They have visible boundaries of separation between the.
Metal and a compound Fe and Fe3C or two compounds Al2O3 and Si2O3 etc. Two component systems are classified based on extent of mutual solid solubility a completely soluble in both liquid and solid phases isomorphous system and b completely soluble in liquid phase whereas solubility is limited in solid state. Here a classic example is the mixture of salt in water.
Its a homogenous mixture of oxygen nitrogen argon carbon. Cu-Ni Ag-Au Ge-Si Al 2 O 3-Cr 2 O 3. Learn how to classify these examples of mixtures below.
For instance a mixture of salt and water a mixture of sugar and water air lemonade soft drink water and so on. A heterogeneous mixture has physically distinct components. The key difference between homogeneous and heterogeneous is that homogeneous materials and mixtures have the same uniform composition and properties throughout whereas heterogeneous materials and mixtures do not have either uniform composition or uniform properties.
Yang et al. Meet the Noble Metals. What Is the Lightest Metal.
This editorial introduces the state of the field along with a collection of Articles. Role of Chemistry in Human Life. Bronze - This common metal is actually an alloy of tin and copper.
Whats the Difference Between Heterogeneous and Homogeneous Mixtures. The alloys that have returned promising results in terms of process-ability by SLM are generally the cast alloys with AlSi10Mg receiving most attention followed by AlSi12. Suspensions heterogeneous A suspension is a mixture between a liquid and particles of a solid.
For isomorphous system - Eg. Developments for their application in powder metallurgy and alloys. Precious Metal Compounds - Homogeneous Catalysts.
This paper briefly reviews some critical aspects of HEAs including core effects phases and crystal structures mechanical. Another example is the air we breathe. Proteomics Reagents and kits.
Mixtures can be either heterogeneous or homogeneous. In practical terms if the property of interest is the same regardless of how much of the mixture is taken the mixture is homogeneous. The steel then becomes heterogeneous as it is formed of two phases the iron-carbon phase called cementite.
Substitutional and interstitial alloys. At least one of the elements mixed is a metal. In heterogeneous catalysis two specific terminologies of atomically dispersed supported metal catalyst ADSMC and single-site heterogeneous catalyst SSHC are easily confused with SACs.
They have no visible boundaries of separation between the constituents. An alloy is a metal that is made by combining two or more metals together. On a small enough scale any mixture can be said to be heterogeneous because a sample could be as small as a single molecule.
These intermetallic alloys appear homogeneous in crystal structure but tend to behave heterogeneously becoming hard and somewhat brittle. This is on the grounds that here the limit among salt and water can. Thus there has been significant interest in these materials leading to an emerging yet exciting new field.
Alloying a metal involves combining it with one or more other metals or non-metals which often enhances its properties. For example sugar or salt dissolved in water alcohol in water etc. This is owing to their good cast-ability alongside low shrinkage due to the large fraction of Al-Si eutectic.
Download the Heterogeneous Mixture - Homogeneous Mixture Worksheet. The prefix hetero indicates difference. Different atomic mechanisms of alloy formation showing pure metal substitutional interstitial and a.
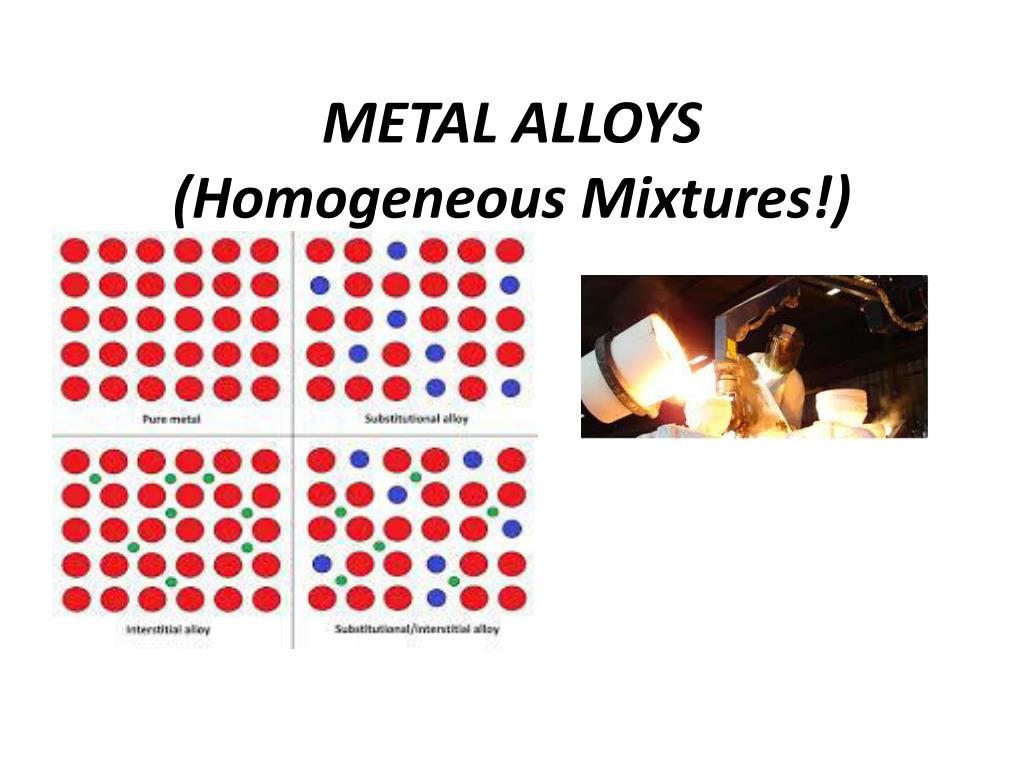
Ppt Metal Alloys Homogeneous Mixtures Powerpoint Presentation Free Download Id 2623682
Is A Copper Penny Homogeneous Or Heterogeneous

Recent Developments Of Heterogeneous Catalysts For Hydrogenation Of Carboxylic Acids To Their Corresponding Alcohols Tamura 2020 Asian Journal Of Organic Chemistry Wiley Online Library

Do Now With You Tablemates Partition Separate The Pictures In The Envelope Into 2 Categories 1 Homogenous Mixture And 2 Heterogeneous Mixture 2 Record Ppt Download
Is An Alloy A Heterogeneous Mixture Quora
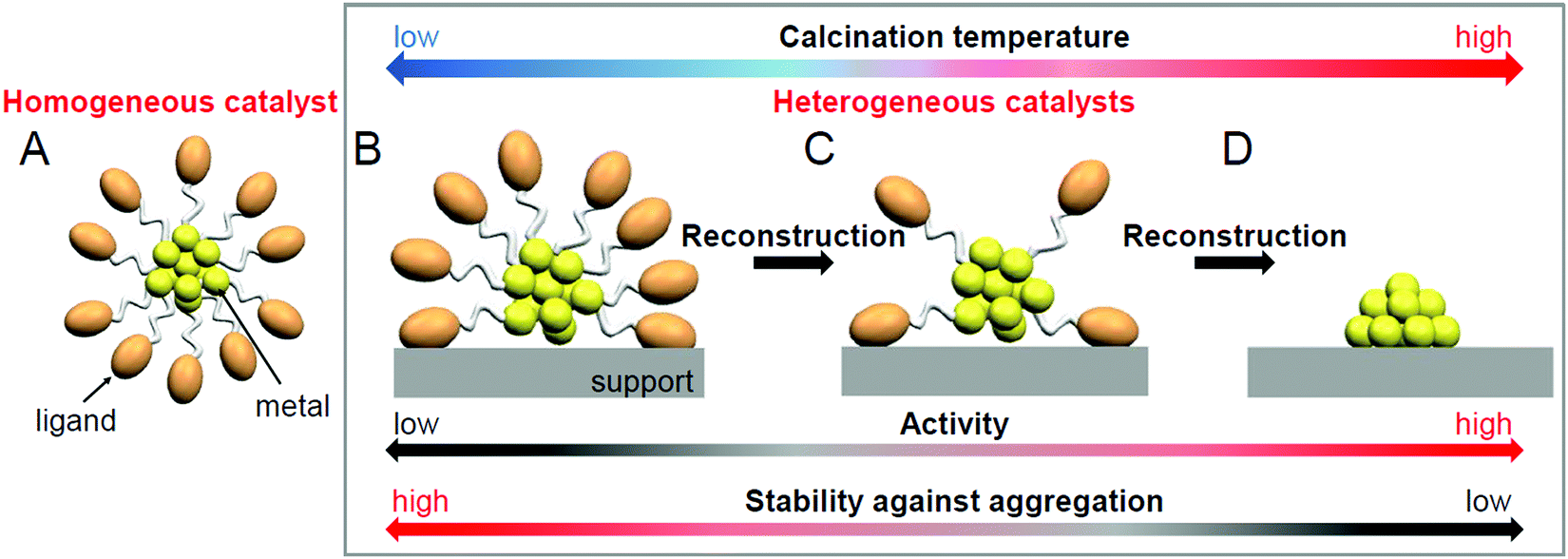
Toward The Creation Of High Performance Heterogeneous Catalysts By Controlled Ligand Desorption From Atomically Precise Metal Nanoclusters Nanoscale Horizons Rsc Publishing Doi 10 1039 D1nh00046b
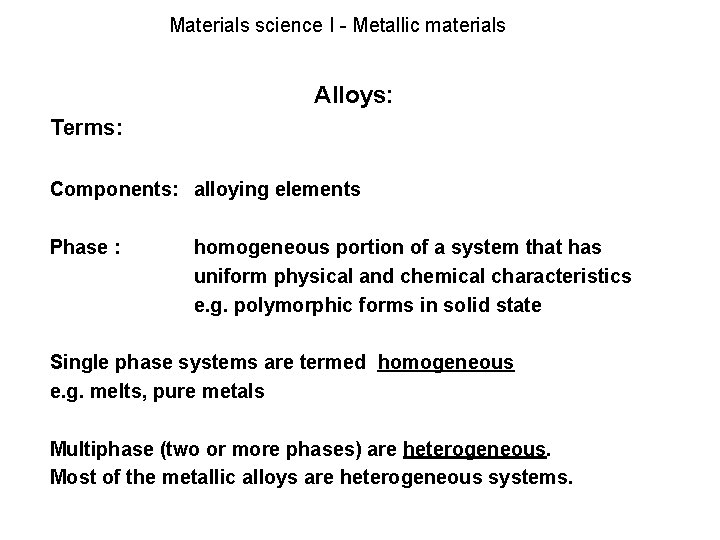
Materials Science I Metallic Materials Solid State Pure

In This Infographic We Have Explained The Importance Of Titanium And Its Industrial Applications Metal

Polar Covalent Bonds The Water Love Story Youtube Covalent Bonding Apologia Chemistry Science Chemistry

Synthesis Of Two Dimensional Metallic Nanosheets From Elemental Metals To Chemically Complex Alloys Yu 2020 Chemnanomat Wiley Online Library

Let S Talk Chemistry All About Mixtures
Difference Between Alloy And Composite Definition Composition Properties And Uses
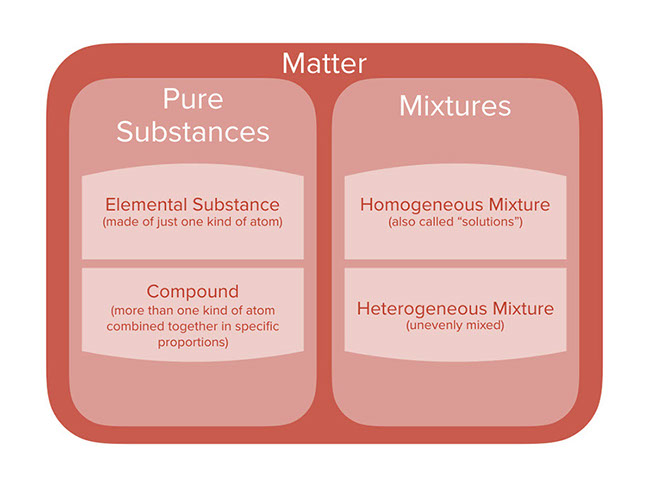
Lesson Categories Of Chemicals And Mixtures

Alloys Are Homogeneous Mixtures Of A Metal With A Metal Or Non Metal Whic Youtube

Chapter 7 Inorganic Materials Properties Of Metals A

Chemistry The Central Science Chapter 23 Section 6



0 Response to "is metal alloys homogeneous or heterogeneous"
Post a Comment